
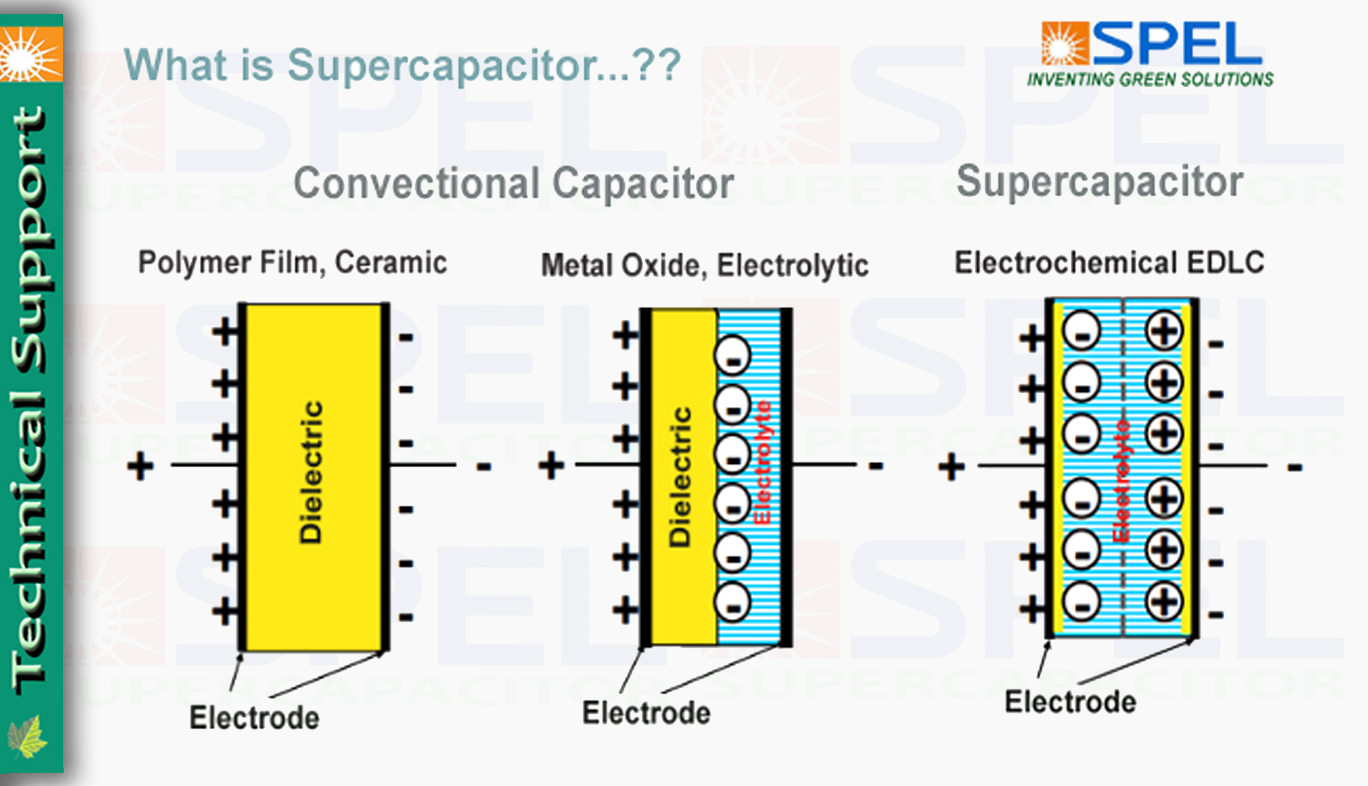
Comparision Convectional Capacitor and Supercapacitor
Supercapacitors can be defined or Classified into different types on basis of storage mechanisim which translates into capacity or a capacitance of supercapacitors, which can be defined as below.
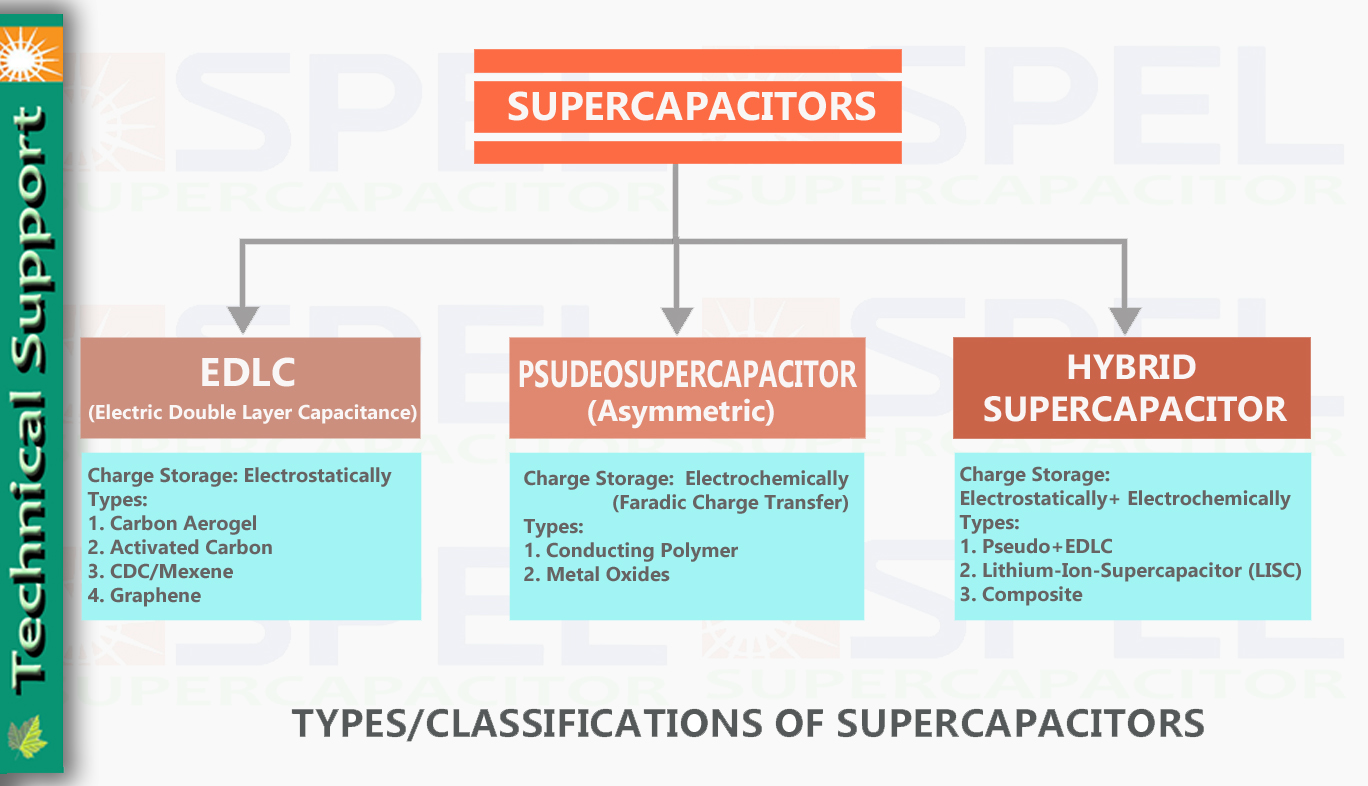
Types and Classification of Supercapacitors based on Storage mechanisim
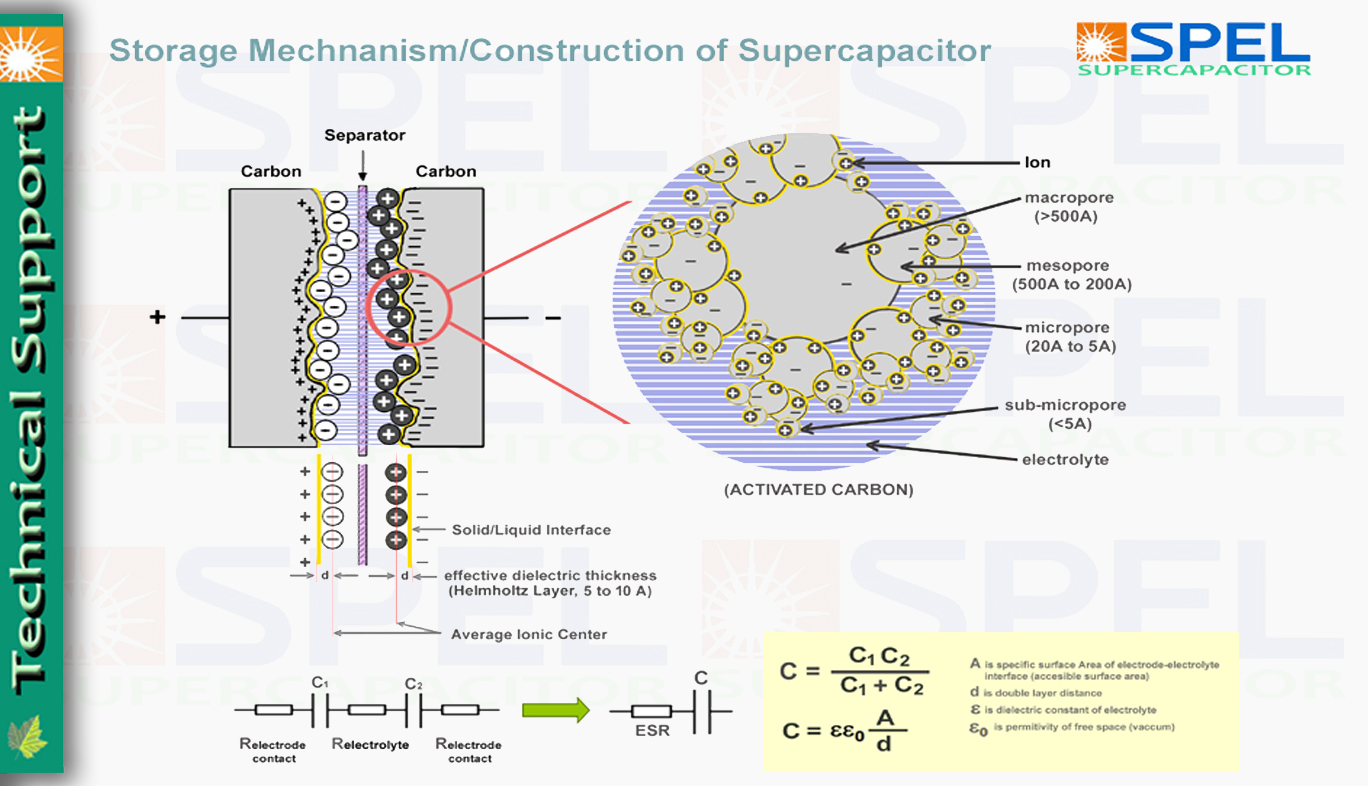
Storage Mechanism of Supercapacitor
Supercapacitors are absoultely safe device unlike batteries when mistreated/abused they do not explode. Unlike Lithium-Ion batteries there is no thermal runaway issues. Above all Supercapacitors are Environment friendly product as no hazardous material is used.
At the basic level Supercapacitors are originally non-polar device. The desiganated anode and cathode are similar or symmetrical type. It is not advisable to change designated pre-defined polarity of these capacitors, there will not be any saftey issues, but would this act will result in increase in ESR of Supercapacitor and would also reduce service life.
In case of Supercapacitors the ESR is of order of milli-Ohms, wheras in case of batteries the ESR is in Kilo-Ohms, due to this batteries when subjected to short circuit or excess current drawing tends to explode due to excessive heating in this scenario, but in case of Supercapacitor due to low ESR maximum drop will occur on load side, resulting in lesser temperature rise in Supercapacitor, moreover construction of supercapacitors incorporate saftey vent, which breaks open when interrnal pressure exceeds 12 bar.
The maximum voltage rating of single cell Supercapacitor is indicated on product, which would normally be like 2.7V/ 2.85V/ 3.0 V per cell. This max rating ofvoltage is mainly derived from the electrochemical stability of the electrolyte and electrode materials.
Utmost care should be taken to "Not to exceed the indicated maximum voltage across Supercapacitor".
Please note if cells are operated above their rated voltage for a long period of time, the life is reduced. This is because of electrolyte breakdown with exposure to high voltage. The amount of damage varies based on the voltage and the amount of time the cell is exposed to the over-voltage condition.
SPEL Supercapacitors are designed to endure temporary high temperature for few minutes, but this would shorten product life. Please note if the product is expose to high temperature over for certain period of time, product shape may get deformed. It is strongly recommend to use product within the specified or indicated maximum temperature limits.
In general failure mode in Supercapacitor is open circuit. In service life Supercapacitors do not have a hard end of life failure similar to batteries. Their end of life is defined as when the capacitance drop is 20% and/or 100% increase in ESR.
By using Arrhenius plots method, logarithim of the chemical kinetics reaction rate constant is plotted against reciprocal of the temperature change to analyze the
effect of temperature on the rates of chemical reactions. It is observrd that for every 10℃ drop of operational temperature, the product life becomes approximately
double if all other conditions being equal. Likewise for every 0.1 V drop, the product life extends about double.