
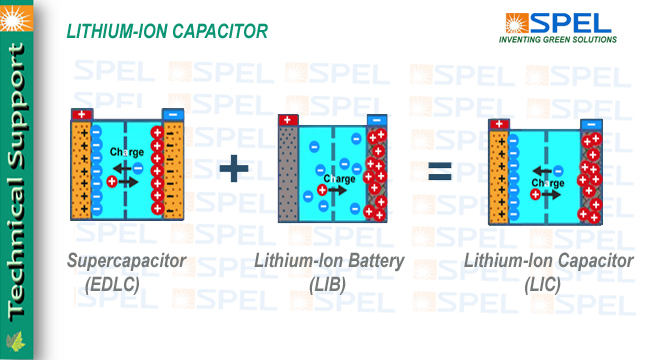
Overview of Lithium-ion Capacitor
As Lithium-ion capacitor is a hybrid electrochemical energy storage device which combines the intercalation mechanism of a lithium-ion battery anode, and the double-layer mechanism of the cathode of an electric double-layer capacitor (EDLC).
Lithium-ion Capacitor (LiC) is combination of "best of both the world" namely EDLCs and Batteries.
Lithium-ion Capacitors are relatively safe when compared with Lithium-Ion Battery, because of composition of electrode material which has minimal Oxide content. Oxide content is like catalyst for heat generation. Also, the internal resistance of LiC is much lower than than LIBs and because of this the internal heat generation is minimal.
Apart from mentioned properties the high specific surface area of LiC electrode enhances the thermal and chemical stability wich makes Lithium-ion Capacitors a safer alternative to Lithium-ion batteries.End of life of Lithium-ion Capacitor is defined as when the capacitance drop is 20% and/or 100% increase in ESR.
By using Arrhenius plots method, logarithim of the chemical kinetics reaction rate constant is plotted against reciprocal of the temperature change to analyze the
effect of temperature on the rates of chemical reactions. It is observrd that for every 10℃ drop of operational temperature, the product life becomes approximately
double if all other conditions being equal. Likewise for every 0.1 V drop, the product life extends about double.